A Graded Oral Food Challenge (GOFC) is the “gold standard” to confirm specific food allergies, or that you’ve reached tolerance following treatment. If your provider believes you are a candidate based on your history, exam, testing, and previous treatment, a GOFC may be recommended.
The Graded Oral Food Challenge is valuable to help answer questions about your treatment progress, whether you should avoiding certain foods, or if you can safely reintroduce a food in your diet.
The Graded Oral Food Challenge will help your provider:
-
Determine how allergic you may be to a specific food
-
Evaluate allergy drop treatment effectiveness
-
Verify you/your child has outgrown a food allergy
The challenge also gives your medical team data that guides how they adjust your treatment.
During a challenge, we test only one food at a time using a detailed protocol and designated food challenge suite that your trained medical team will closely monitor. We will give you the specified food in timed intervals at pre-calculated amounts.
Our challenge protocol integrates the best practices from leading food allergy centers that provide food challenges. Our team and facility are equipped with state-of-the art technology and training to address the many facets of food challenges in a safe, comfortable environment.
Talk with your provider at your next visit for details about the Graded Oral Food Challenge and whether you might be a candidate.

Graded Oral Food Challenge Reports
April 2025 GOFC Report
Allergy Associates of La Crosse initiated a Graded Oral Food Challenge (GOFC) program in spring 2016.
The objective of challenges, which typically take 3-4 hours, is to eat a full serving of a food in graded increments, with detailed patient observations before, during, and after the challenge.
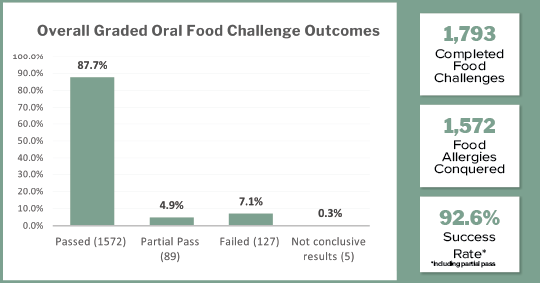
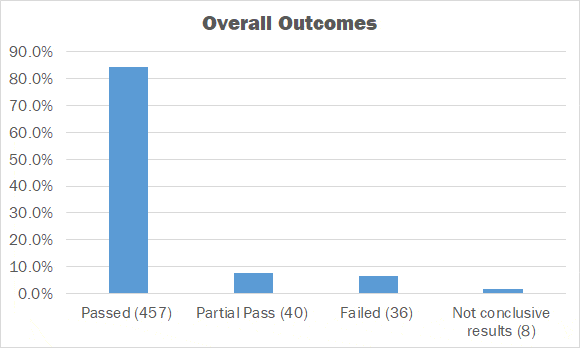
As of spring 2025, a total of 1,793 challenges have been performed that provide the data for this report.
• Passed = 87.7%
• Partial Pass = 4.9%
• Failed = 7.1%
• Not conclusive results = .3%
When including partial pass challenges into the results, an impressive 92.6% success rate has been achieved.
Patient selection is driven by detailed metrics of their conditions (comorbidities), history of testing and treatment including: Specific IgE, Total IgE, skin prick testing, treatment duration/level, history of severe reactions, several other measured criteria, and provider judgment.
Key Report Findings
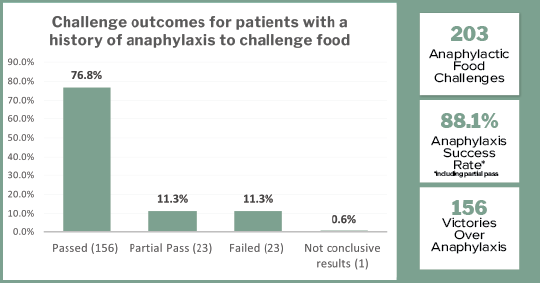
A KEY finding of our program is the large number of patients with a history of food anaphylaxis (n=690 or 38.5% of all challenges):
- 86.8% of the patients with any history of anaphylaxis passed their challenge
- 4.9% partially passed
- 8.1% failed
Of the 690 with anaphylaxis, 203 patients were challenged directly to their anaphylactic food (n=203):
- 76.8% passed their challenge
- 11.3% partially passed
- 11.3% failed their challenge
General results of the program challenges:
Basic demographics of challenge patients:
- Age: Average patient age = 14 years. The youngest is 2; oldest is 77.
- Gender: 1167 Males, 626 Females
- Average years of sublingual immunotherapy at time of challenge: 5.59 years
Most Common Foods Tested:
- Peanut — 234
- Almond — 229
- Hazelnut — 180
- Cashew — 172
- Walnut — 163
- Egg (cooked) — 116
- Pecan — 99
- Egg (baked) — 97
- Milk — 77
- Pistachio — 73
The Toughest Nuts to Crack
Tree nuts like cashew, pecan, and walnut are some of the hardest food allergies to overcome — especially for patients who have experienced anaphylaxis.
here’s the good news:
92.4% of our tree nut challenge patients successfully passed their graded oral food challenges!
Even the toughest nuts to crack can be helped — with the power of personalized sublingual immunotherapy.
Safety Statistics
From a safety standpoint in all challenges, epinephrine use occurred six times (0.33%) in 9 years. No patients required or reported follow-up at an Emergency Center, and no patients were admitted to a hospital.
Request an Appointment